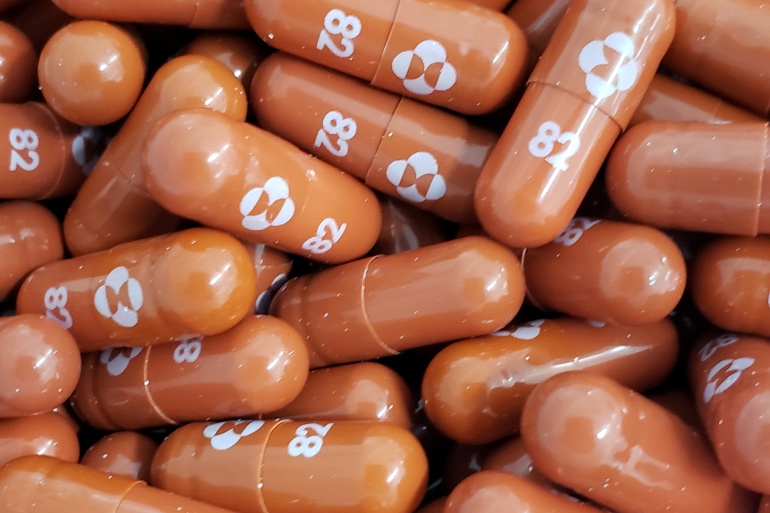
On Thursday, the UK Medicines and Healthcare Products Regulatory Agency said Merk and Ridgeback Biotherapeutics’ oral antiviral, molnupiravir, for the treatment of mild-to-moderate Covid-19 had been authorized. The oral antiviral, which is used to treat adults at risk for severe illness, is the first antiviral to be authorized anywhere in the world for the treatment of Covid-19. The drug, which comes in capsule form, will be known as Lagevrio.
While the European Medicines Agency is deliberating its marketing application, Merk and Ridgeback, in an announcement last week, stated that the capsule reduced the risk of hospitalization or death by Covid-19 by half.
“At the interim analysis, molnupiravir reduced the risk of hospitalization or death by approximately 50%; 7.3% of patients who received molnupiravir were either hospitalized or died through Day 29 following randomization (28/385), compared with 14.1% of placebo treated patients (53,377),” the company said in a news release in October .Through Day 29, no deaths were reported in patients who received molnupiravir, as compared to 8 deaths in patients who received placebo.”
Meanwhile, the Companies have requested the United States Food and Drug Administration emergency use authorization for the drugs, and the FDA has stated that it will hold its Advisory Committee on Anti-Epidemiology on November 30 to discuss the ability of Minopbiriver to deal with mild-to-moderate Covid-19 in adults who are at high risk, including those facing the possibility of hospitalization or death.
Speaking on the invention, former commissioner of the U.S. Food and Drug Administration, Dr. Scott Gottlieb, told CNN reporter Anderson Cooper that the antiviral was the most impactful result of an oral drug he remembers seeing available for the treatment of a respiratory pathogen.


2 comments
This is good news
Nice